Drug Metabolism & Pharmacokinetics (DMPK)
Comprehensive Drug Metabolism and Pharmacokinetics (DMPK) services, applying state-of-the-art techniques and best-in-class approaches to generate data for critical milestones and decision-making.
Download Fact Sheet
Proven techniques and best-in-class approaches
Our DMPK scientific staff provides comprehensive Drug Metabolism and Pharmacokinetics (DMPK) services, applying state-of-the-art techniques and best-in-class approaches to generate data for critical milestones and decision-making during drug discovery and development.
We provide broad and in-depth expertise/advice to clients on appropriate study designs, execution of studies, and interpretation of the data.
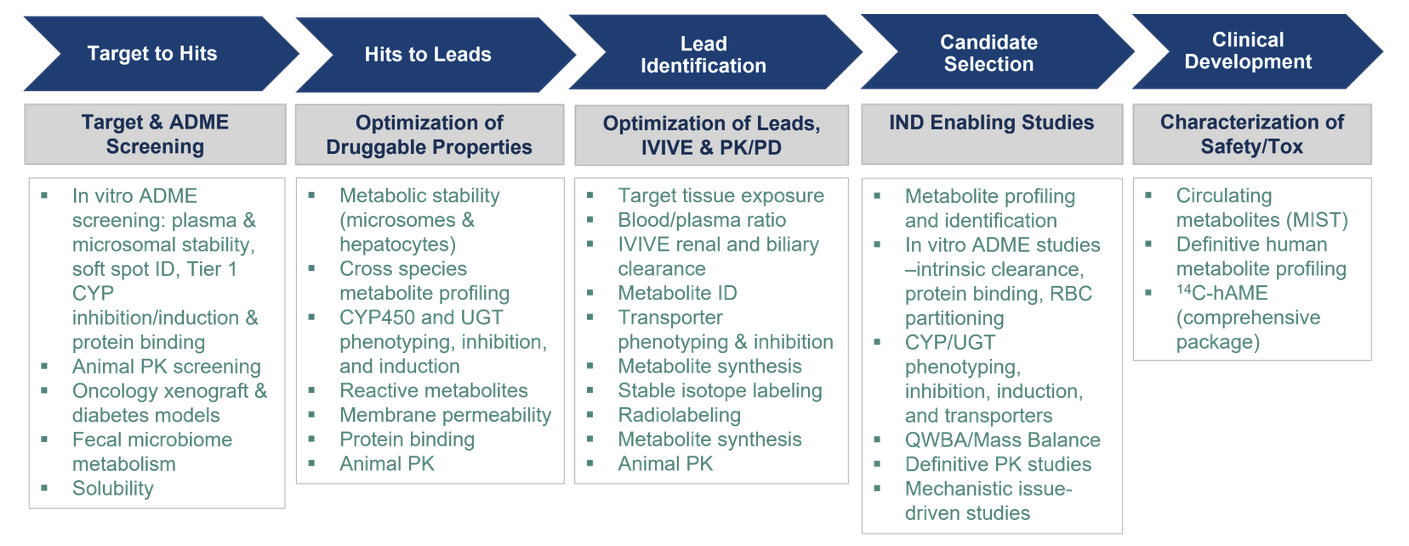
We offer extensive drug metabolism and pharmacokinetic capabilities for new chemical entities and compounds in development. We provide rapid turnaround of high-quality key DMPK data sets to support critical decisions in advancing potential therapeutic agents for further development.
A range of capabilities and experience exist at Frontage to support the discovery and development of drug candidates.
Full Service Radiolabeled Human 14C AME (hAME) Studies
Experience the benefits and added value of Frontage’s complete suite of clinical and analytical services for hAME studies.
Learn moreResources To Consider


IND Enabling Services Brochure

MIST Application Note

Accurate Mass: The best solution for metabolite identification in…

Metabolites in Safety Testing (MIST): Analytical Strategy