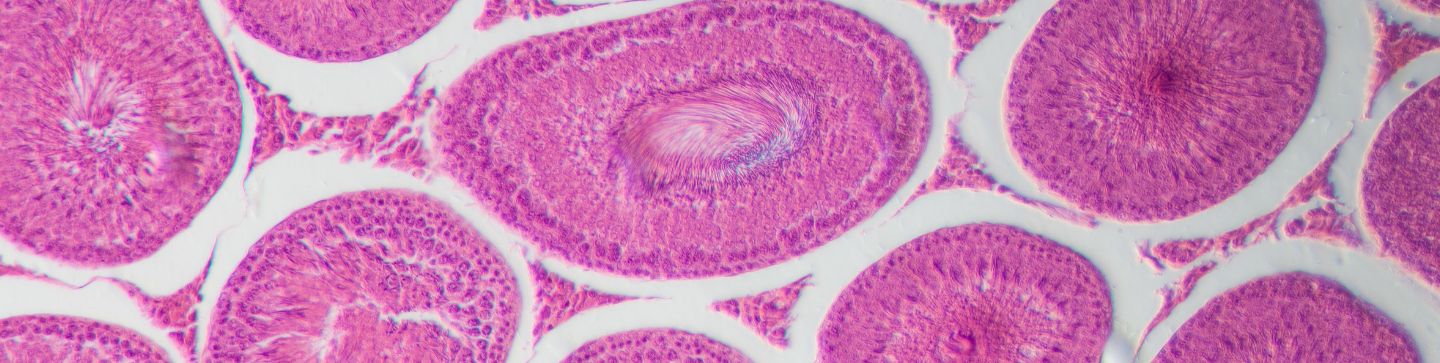
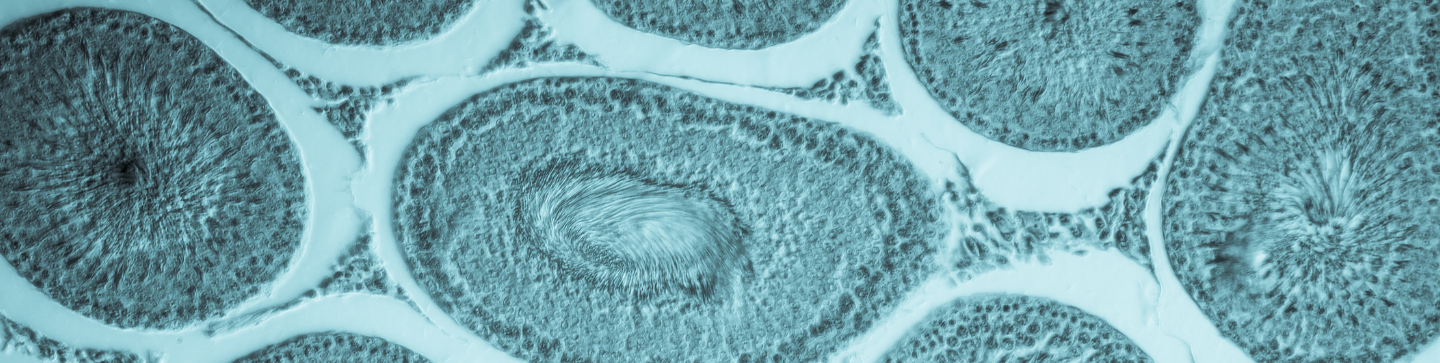
Toxicology
Specialized Toxicology Services
 Services & Solutions
/
Safety Assessment & Toxicology
/
Toxicology
/
Specialized Toxicology Services
Services & Solutions
/
Safety Assessment & Toxicology
/
Toxicology
/
Specialized Toxicology Services
Qualifying your drugs for NDA submissions
Frontage’s centrally-located safety and toxicology group has over 20 years of experience conducting toxicology testing and research. At Frontage, we provide comprehensive coverage by offering a wide selection of test species and multiple drug administration routes, to help prepare your compounds for IND and NDA submissions.
Drive your drugs forward with specialized toxicology studies.
Specialized Toxicology Services at Frontage
- DART: Development and reproductive toxicology (DART) studies are critical studies that usually take place after IND and before NDA submissions, and help assess the effects of a drug on the entire reproductive process. Frontage offers the following DART studies:
- Embryo-Fetal Development (EFD) Studies in Rats and Rabbits
- Fertility and Early Embryonic Development (FEED) Studies in Rats
- Pre- and Postnatal Development (PPND) Studies in Rats
- Single and Multi-generation studies in Rats
- Sperm Analysis/Vaginal Cytology
- Juvenile Toxicity Studies in Rats and Dogs
- Infant Nutrition Studies in Pigs and Rats
- Chronic Toxicity Studies: Chronic toxicity studies help in determining the toxicity profile by noting the adverse effects of a drug after repeated or continuous administration of the drug. Frontage provides comprehensive, high-quality study reports supporting your drug at later stages of development.
- Investigative Toxicology Studies
- Neurotoxicity Studies in Rats: Neurotoxicity studies help understand the effects of a pharmaceutical on the functions of the nervous systems, such as motor activity, functional observational battery, learning and memory paradigms, and startle reflexes.
- Carcinogenicity Studies in Rats and Mice (including transgenic): Carcinogenicity studies are used to determine the tumorigenic effects of chronic exposure to a compound in humans and are typically required for compounds that are expected to be used long-term. Conducting carcinogenicity studies is a critical step to take before the drug in question is pushed for market approval. Frontage offers two types of carcinogenicity studies:
- Routine Carcinogenicity Study: This is the conventional study exposing the study species to the compound for two years.
- Transgenic Carcinogenicity Study: This is a shorter study, usually 6 months long, performed on models that are specifically genetically engineered for carcinogenicity studies.
Contact us for your specialized toxicology studies.

GLP-Compliant Toxicology Services
Our facility, located in Chicago, Illinois, provides full service, GLP-compliant toxicology and related non-clinical development services supporting the pharmaceutical and biotechnology industries.
Developmental and Reproductive Toxicity (DART) Services
Frontage has a highly trained technical staff and expert scientists with a long history of successfully performing regulated DART studies.
Safety and Toxicology Services
Frontage Laboratories provide a variety of safety assessment and toxicology services to guide new therapies from discovery through development to product launch.
Resources To Consider


Toxicology Services
DART Fact Sheet
Guidelines for Developmental and Reproductive Toxicity (DART) Testing