fact-sheet
In Vitro Cardiac Safety Evaluation
The FDA and the ICH S7B provide guidelines for assessing cardiac safety. In Vitro Assays are the key strategies in cardiac safety evaluation. These assays involve the use of cell lines or isolated hearts to assess the direct effects of drugs on cardiac ion channels, particularly the hERG channel, which is associated with the QT interval prolongation.
The Comprehensive In Vitro Proarrhythmia Assay (CIPA) is an example of a modernized screening paradigm that assesses multiple ion channels and cardiomyocyte function. Manual patch clamp is the gold standard for ion channel detection methods.
Register to gain access to gated resources.
"*" indicates required fields
Resources to Consider


poster
IN Vitro Assessment of the inhibition of Human Organic Anion...
The present study was aimed to validate this alternate approach to assess inhibition of or...
fact-sheet
In Vitro ADME Services
Frontage Global Drug Discovery Services (GDDS) offers a full spectrum of in vitro ADME ass...

podcast
In Vitro BE Study in ANDA Drug Product Development
In this podcast, we talk about an in vitro bioequivalence (BE) Study in ANDA drug developm...

poster
Stability Study for EMLA Cream Using In Vitro Percutaneous Absorption
EMLA Cream (lidocaine 2.5% and prilocaine 2.5%) is an emulsion in which the oil phase is a...

podcast
Importance of Safety Pharmacology in IND Filings
In this podcast, we discuss the importance of understanding the role of Safety Pharmacolog...

webinar
Metabolites in Safety Testing (MIST): Analytical Strategy
In this webinar, Dr. Philip Tiller reviews Metabolites in Safety Testing (MIST) and discus...

poster
AACR 2023: Evaluation of a 293 gene oncology-focused exome sequencing...
Frontage Genomics Services has validated a custom-designed 293 cancer-related genes panel....

journal
Gemogenovatucel-T Advantage in Clonal Tumor Mutation Burden–High...
In a recent clinical study, Gradalis’ Vigil showed a significant survival benefit in cTM...

journal
Population pharmacokinetics of caspofungin in critically ill Chinese...
Frontage supported a recently published prospective PopPK study on caspofungin in critical...

poster
EBF 2025: A generic immunogenicity assay for the detection of...
Discover how Frontage developed and qualified a generic ECLIA bridging immunogenicity assa...
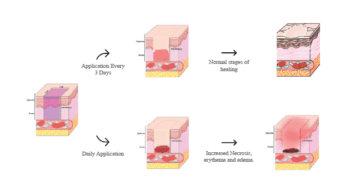
poster
ACT 2025 Study Design Considerations for Wound Healing Research in...
Discover how Frontage’s Safety & Toxicology team optimized wound healing study design in...



