webinar
Guidelines for Developmental and Reproductive Toxicity (DART) Testing
This webinar will provide a general overview of the regulatory guidelines required for developmental and reproductive toxicity (DART) testing. In order to initiate clinical trials for women of child-bearing potential (WOCBP), the FDA requires initiation of preclinical DART studies using mammalian research models. These regulated studies provide important information regarding the effects of drug exposures prior to and during parental mating, as well as evaluating maternal and fetal changes during gestation, and identifying any alterations in the development of the offspring following birth.
Register to gain access to gated resources.
"*" indicates required fields

Resources to Consider


white-paper
Testing of Carryover & After MRD Benchtop Stability for...
In this white paper, we show testing of two critical parameters for the method validation ...

webinar
Microbiological Testing Considerations for Pharmaceutical Products
In this webinar, speaker Christopher Gilmer introduces microbiological testing for raw mat...

white-paper
Outsourcing Analytical Testing For Biologics – A CRO’s...
In this white paper, we provide an overview of how CROs can support the analysis of biolog...

fact-sheet
CMC GMP Analytical Testing for New Chemical Entities and Commercial...
Ensure comprehensive product analysis with Frontage’s team of experienced analytical sci...

fact-sheet
CMC GMP Analytical Testing for Biologics
Due to their size, nature, and biological origin, biologics or biopharmaceuticals are inhe...

poster
EBF 2025: A generic immunogenicity assay for the detection of...
Discover how Frontage developed and qualified a generic ECLIA bridging immunogenicity assa...
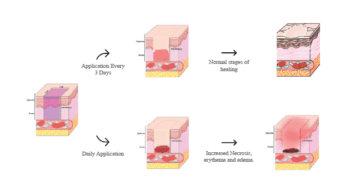
poster
ACT 2025 Study Design Considerations for Wound Healing Research in...
Discover how Frontage’s Safety & Toxicology team optimized wound healing study design in...

poster
AAPS 2025: Establishing Linear Viscoelastic Region and Gelation...
By defining the linear viscoelastic region and gelation dynamics, this work provides key i...

Duplex Sequencing: A Powerful Tool for In Vivo Gene Mutation
Discover our EMGS 2025 poster showcasing how Duplex Sequencing (ecNGS) reveals the genotox...
poster
AAIC 2025: Comparison of Plasma pTau217 Assays on Different Platforms
Plasma pTau217 was recently discovered as a prominent blood-based biomarker in the early d...