From Paper to Cloud: How Frontage Digital Lab is Redefining Compliance and Efficiency

Meet the Author

Dr. John Lin is a seasoned leader in the life sciences industry, dedicated to advancing innovation, compliance, and operational excellence across the CRO sector. As Co-CEO of Frontage Laboratories, he has spearheaded numerous strategic initiatives, including the company’s digital transformation journey. John believes in harnessing technology to not only improve efficiency but also to enhance scientific rigor and client collaboration. His vision continues to drive Frontage’s mission of accelerating drug development from discovery through product release.
In today’s regulated bioanalytical landscape, paper-based systems are no longer just inefficient — they’re a liability. Archive rooms packed with binders, files scattered across departments, and manual recordkeeping slow study timelines and increase compliance risks.
At Frontage Laboratories, we saw the need for change. To deliver greater compliance, efficiency, and client value, we launched a multi-year transformation to build a fully integrated, paperless bioanalytical framework.
The Vision: A Paperless, Future-Ready Lab
Our vision was clear: create a digital ecosystem that ensures data integrity, accelerates reporting, and strengthens compliance — all powered by a centralized digital data center. This secure, scalable hub doesn’t just store information; it lays the foundation for AI-assisted applications by enabling structured, high-quality data ready for advanced analytics and innovation.
The Journey: From Electronic Lab Notebook (ELN) to AI-Powered Insights
Our digital transformation unfolded in key milestones:
- 2018: IDBS informatics platform launched at Frontage with method registration templates
- 2020: StudyDoc deployment for automated reporting
- 2023: Integrated Electronic Binder (IEBS) integration for full paperless binder workflows
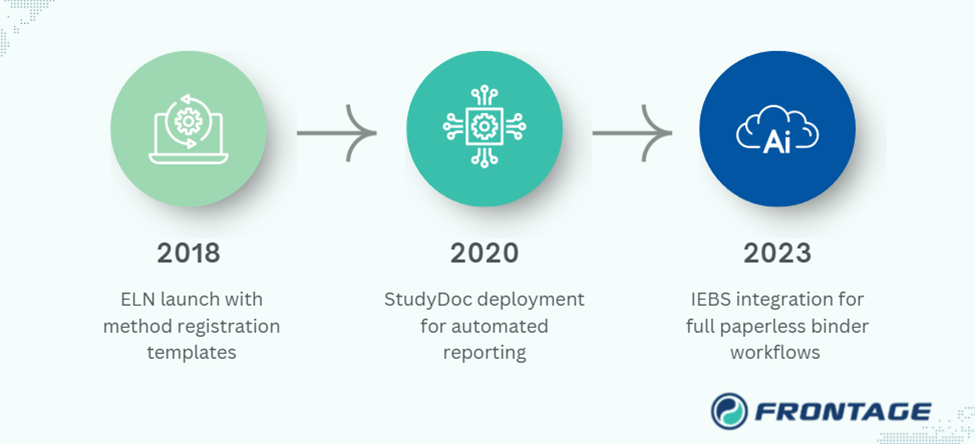
Along the way, Frontage implemented key customizations such as direct balance weight capture, study-specific traceable reports, and incurred sample tracking with full chain of custody.
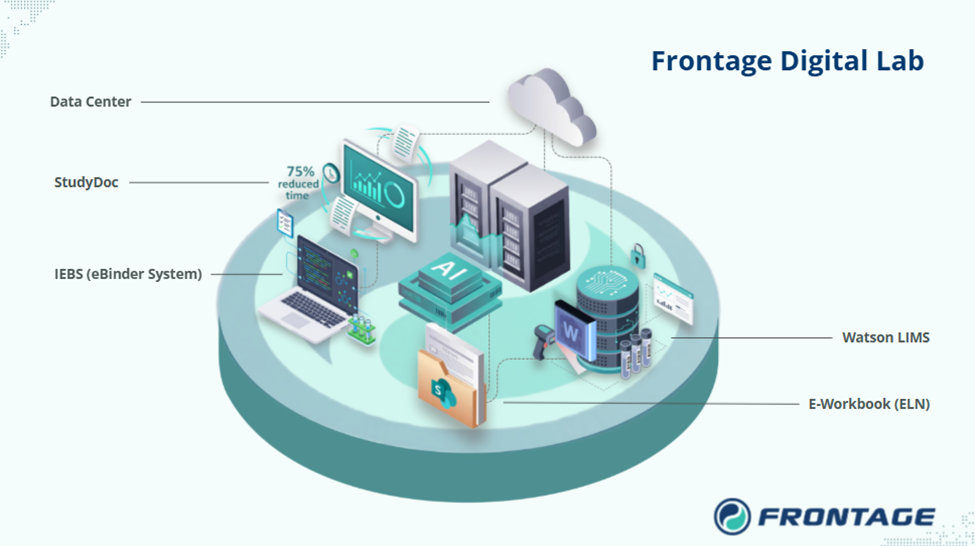
As a result, a unified framework integrates best-in-class tools all working together, supported by a centralized digital data center:
- Data Center: The backbone of our digital ecosystem — centralized, compliant, and scalable storage that transforms raw study data into AI-ready assets, ensuring long-term integrity and enabling advanced applications like predictive modeling and AI-powered QC.
- E-Workbook (ELN): Structured templates, catalog, and experiment workflows.
- Watson LIMS: Secure backbone for sample and data tracking.
- StudyDoc: Automated reporting that cut turnaround time by 75%.
- IEBS (eBinder System): SharePoint-based binders with automated approvals and archiving, replacing physical study binders.
The Benefits: Why Digital Matters for Clients
Our transformation was not just about internal efficiency — it delivers direct value to our partners:
- Compliance: Secure versioning, audit trails, immutable records
- Efficiency: Automated QC, reduced manual workload, faster reporting
- Collaboration: Centralized, globally accessible records with sponsor transparency
- Future-Readiness: BI dashboards, AI-powered QC, and scalable cloud architecture for next-generation applications
The Impact: Leading the CRO Industry in Digital Innovation
By implementing this paperless framework centered on our digital data center, Frontage strengthens scientific rigor, accelerates client delivery, and ensures regulatory readiness. More importantly, it positions us as a leader in digital innovation within the CRO industry — building a foundation for AI-driven analytics, predictive modeling, and beyond.
Conclusion: Partnering for the Future
Frontage’s digital transformation is about more than compliance — it’s about preparing for the next generation of drug development. By investing in a fully integrated, paperless lab framework, we ensure that our clients gain faster insights, stronger data integrity, and the confidence of working with a partner committed to innovation.
We are proud to lead the way in digital bioanalysis — and excited to help our clients move from drug candidates to therapeutics with speed, compliance, and trust.
Learn more about our services:

Bioanalytical – Small & Large Molecules
Integrated Bioanalytical Solutions for Complex Scientific Challenges

Genomics
Frontage offers a comprehensive portfolio of genomics services to meet the needs of our clients

Central Lab Services
Next Generation Central Lab Services
Biomarkers & Precision Medicine
Experienced in developing, qualifying, and validating biomarker assays