Frontage Europe – Milan
The strategic decision to open the site in Europe represents a step forward to increasing Frontage efficiency and streamline processes. Being close to our clients enables us to meet unique needs, shape relationships and generate more opportunities for collaboration.

Based on the principle of global reach and regional touch, in 2024 Frontage Laboratories Inc. expanded its business in Europe through its fully owned subsidiary Frontage Europe.
Frontage Europe supports you to progress your preclinical and clinical research pipelines.
In our 2,500 square meters facility in Milan, Italy, we provide bioanalysis of small & large molecules, isotope chemistry & metabolite identification and central lab services.
The laboratory features state-of-the-art equipments for quantitative mass spectrometry, ligand-binding and biomarker assays. In-house capabilities for stable-labeled internal standard synthesis for quantitative analysis are available. Expertise on pharmacokinetics and scientific advice on the preparation of regulatory documents (e.g. IB, IND) are our value-added service.
The process of bringing a drug to market requires both scientific knowledge and strict compliance with regulatory requirements. We operate under Good Laboratory Practice (GLP) quality system and comply with Good Clinical Practice (GCP) to support clinical studies. At Frontage Europe bioanalytical assays validation and sample analysis are performed in compliance with the international ICH M10 regulatory guidance. Quality is assured by rigorous implementation of SOPs aimed at enhancing consistency, reliability and increasing process efficiency.
We consider experience and knowledge retention to be key factors for business continuity. Our staff has on average more than 15 years of service in a CRO environment and previous experience in leading pharma companies. By engaging with other teams and departments at a global level, we develop competencies, improve critical-thinking skills and learn from multiple case studies.
If you are interested in learning more about our capabilities or getting a quote, please contact us at sales@frontagelab.com

Bioanalytical – Small & Large Molecules
Integrated Bioanalytical Solutions for Complex Scientific Challenges

Central Lab Logistics
A robust set of standardized procedures ensures consistency across global studies
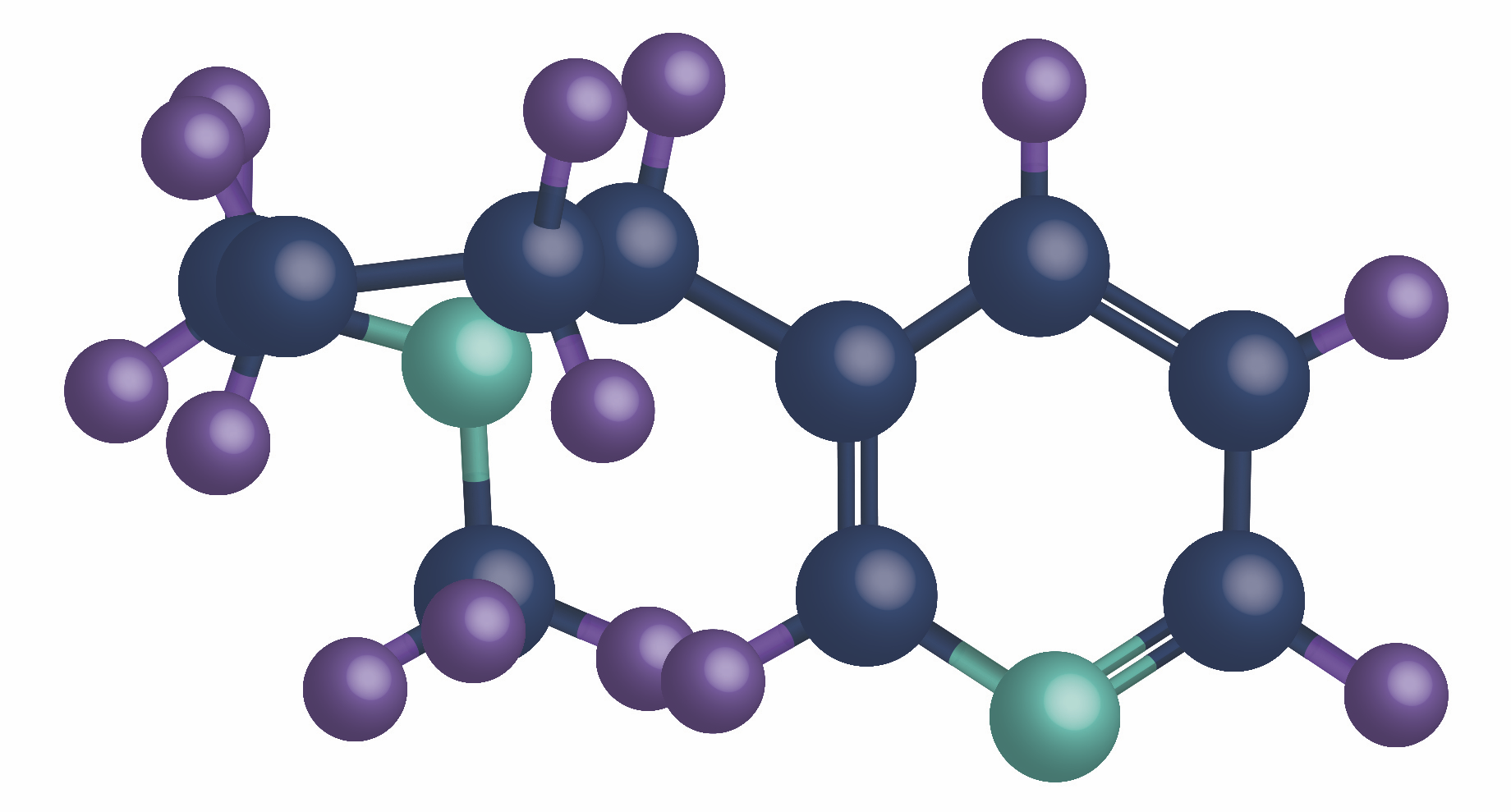
Radio Chemistry & SLIS Synthesis
Expertise in radiolabeled and stable‑labeled compounds

Metabolite Identification
A key in supporting the development of your drug candidate

Pharmacokinetics (PK)
Evaluating pharmacokinetic parameters is essential for determining drug dosing and frequency
A range of capabilities and experience exist at Frontage Europe to support the discovery and development of drug candidates
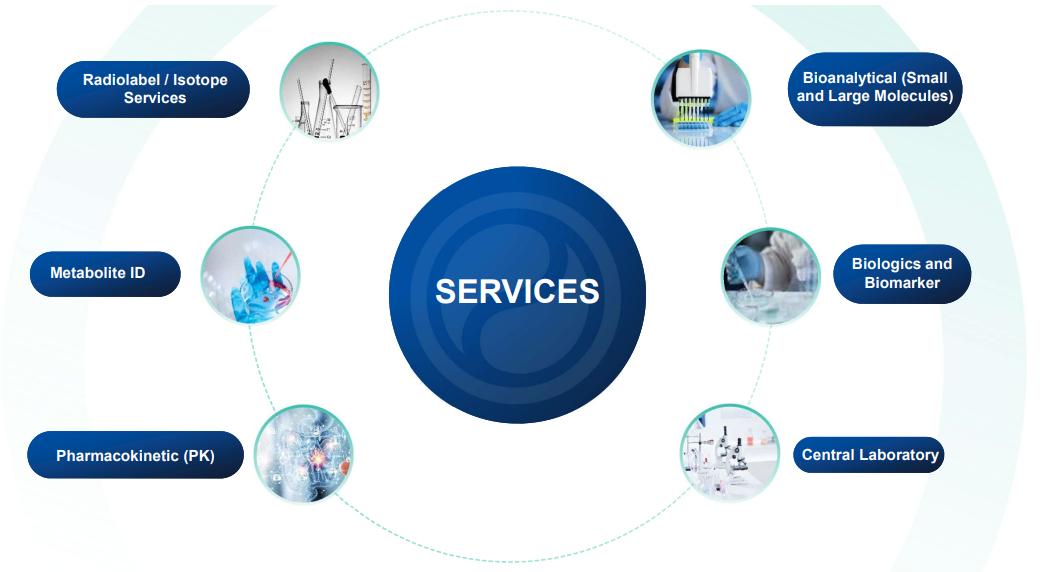
Reduce the risk of failures by comprehensive integrated services.
Product development involves multiple disciplines and deep understanding of the whole process. We offer scalable solutions to ensure each client receives the support needed to advance its products along the pipeline.
Learn moreResources To Consider


EBF 2025: A generic immunogenicity assay for the detection of…
AAIC 2025: Comparison of Plasma pTau217 Assays on Different Platforms

Accurate Mass: The best solution for metabolite identification in…