Who We Are
We are a CRO providing integrated, science-driven, product development services throughout the drug discovery and development process to enable life science companies to achieve their drug development goals. We have enabled many innovators, generic, and consumer health companies of all sizes to file IND, NDA, ANDA, BLA, and 505(b)(2) submissions in global markets allowing for the successful development of important therapies and products for patients. We are committed to providing rigorous scientific expertise to ensure the highest quality and compliance. We have successfully assisted clients in advancing hundreds of molecules through development to commercial launch in global markets.
Two Continents, One System
We benefit greatly from having operations in both North America and China (the two largest markets for CRO services in the world) and are well-placed to capture growth opportunities in both markets. Our “Two Continents, One System” approach is integral to our commitment to high-quality standards. This approach assures our customers the same quality standards, operating procedures, and systems in both China and North America, whilst also providing our customers with a detailed and highly experienced understanding of the regulations and requirements for drug discovery and development in both countries. This approach enables us to be a partner of choice for companies with multinational requirements or that need support for parallel submissions with the US FDA and China FDA.
The Services We Offer
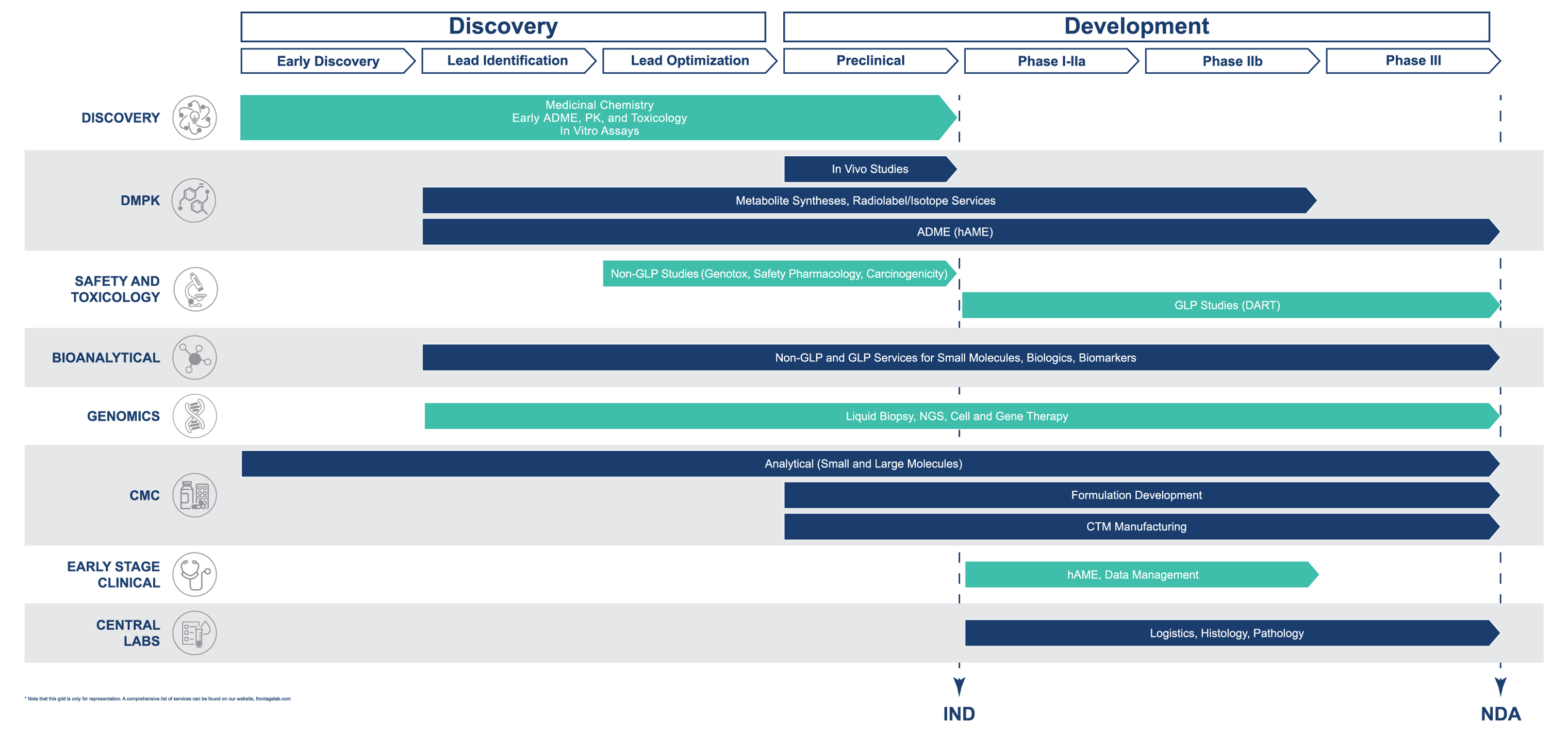
Drug Candidate Evaluation Solutions
- Organic Chemistry
- Drug Metabolism and Pharmacokinetics (DMPK)
- Safety and Toxicology
- Bioanalytical Services
- Genomics Services
Product Development and CMC Solutions
Central Laboratories and Testing
The Value We Provide
We are a value-added partner focusing on solving our customers’ most significant and complex drug discovery and development challenges. Frontage’s scientific knowledge base, technical expertise, and reputation for high-quality services have been integral to our ability to enter into long-term solid strategic relationships and partnerships with our key customers.
- Technology: World-class facilities and equipment that remain at the forefront of the global pharmaceutical research, analytical, and development standards
- Quality: Stringent quality management systems and a strong track record of regulatory inspections
- Expertise: Proven ability to deliver value-add technical expertise because of our deep pool of talented scientists and highly experienced management team.
We are a full-service CRO specializing in collaborations with pharmaceutical & biotech companies to help them bring drug candidates to market.